|
|
马上注册,精彩即将继续...
您需要 登录 才可以下载或查看,没有帐号?立即注册
x
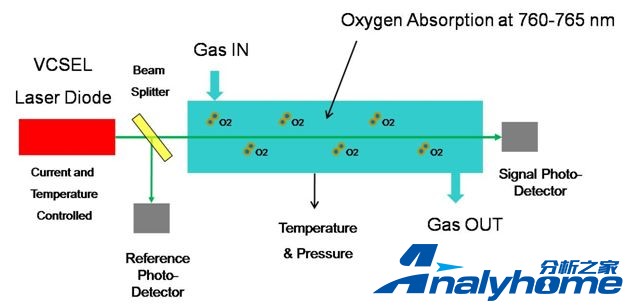
Oxigraf oxygen sensor uses absorption spectroscopy to measure the concentration of oxygen molecules in a gas sample. Absorption spectroscopy for oxygen occurs in a region of the visible spectrum near 760 nm where no interferences by other gases are known. The emission line width of the laser diode source used by Oxigraf and the absorption width of the individual electronic-rotational lines of O2 are very narrow, both less than 0.01nm. To measure oxygen the spectrally pure laser is precisely tuned thermally and electronically to an oxygen absorption line. The particular line to be used is chosen for its absorption strength and for its spectral match to the laser’s output wavelength. As the oxygen concentration increases in the sample chamber, the light intensity, measured with a photodiode detector, is attenuated by the energy absorbed by oxygen molecules. The oxygen analyzer response varies linearly with oxygen concentration.
Oxygen Sensor Operation -
The laser wavelength is controlled by two (2) methods, by temperature control and by current control of the laser diode. Precise control allows the laser wavelength to be varied across an oxygen absorption line. These methods are described below:
Temperature control
A thermoelectric device and thermistor precisely regulate the temperature of the laser diode. The thermal mass of the laser mount results in a long thermal time constant and slow response time for this control loop.
Current Control
Fast control of laser wavelength is done by modifying laser current. The laser current varies the output wavelength stepwise to provide multiple absorption samples. Samples are made at a baseline selected away from the absorption peak, at the absorption peak itself, at the half-height or shoulders of the line, and at intermediate points during each sample cycle.
The baseline or zero sample gives a measure of the optical attenuation with no oxygen absorption. The shoulder samples are forced to be equal in amplitude by adjusting the laser current thus centering or “locking” the laser emission wavelength to the oxygen absorption wavelength. This also provides a measurement of the line width allowing corrections to be made for pressure or collision broadening. Finally the integral of the absorption peak less the average baseline is used as a measure of oxygen absorption. Because of the baseline correction, the oxygen concentration measurement is not affected by attenuation due to dirt on the cell windows or laser aging.
The Oxigraf stepped-laser-drive waveform has numerous advantages.
Every period contains a zero oxygen correction and a line-centering function.
Every period also contains a measure of absorption integrated across the line width.
All the functions can be accomplished on a single, low cost module with board-mounted sample cell for a unit cost in quantity of about $2000.
Oxigraf technology is protected by several patents referenced below.
Laser Mode Structure
Low cost laser diodes, using temperature scanning, typically have narrow regions of linear wavelength interrupted by abrupt wavelength change caused by mode hopping every 0.1 nm or so. Oxigraf has adopted a Vertical Cavity Surface Emitting Laser (VCSEL) diode, which is single mode over at least 1 nm. The mean lifetime of these laser diodes is estimated to be 500,000 hours. The slope of single mode operation is 0.07 nm/C; over a 20o C thermoelectric tuning range, there are usually 4 to 10 oxygen lines available in the range from 760 to 765 nm. The oxygen line width and the laser emission bandwidths in these units are roughly 0.003 and 0.0003 nm, respectively. To find a usable oxygen line, Oxigraf software performs an automated search process. The lines are automatically evaluated and only the best one is selected. Also, during calibration the software corrects for any changes in the temperature set point. Drift can occur as the laser diode and other components age.
Temperature Control and Compensation
Regulation of the temperature of the gas sample minimizes the need for corrections to be made to the oxygen measurement. At room temperature the error may be positive or negative and as large as 1% per oC. To minimize errors the sample gas is heated to about 45oC; this also prevents condensation of water onto the optics when water is in the sample. Additional corrections are made to the measurement during warm-up and other times when the sample is not at 45o. The temperature compensation factor is only good for the absorption line selected but will be constant for the life of the sensor. The reference cell is also heated and maintained at a constant temperature.
Pressure Correction
Pressure is monitored inside the sample chamber with a pressure transducer. Changes in pressure due to barometric changes or pneumatic variations in the sample circuit are corrected in real time. The pressure of the sample at time of measurement is also needed to convert from partial pressure to per cent oxygen. In addition, during low pressure applications, as would occur at high altitude, the pressure measurement is used together with an empirical look-up table to compensate for collision and Doppler broadening over the pressure range of 15 to 120 kPascal. An optional pressure range from 15 to 200 kPascal is available.
Cross Sensitivity
Cross sensitivity refers to errors caused by a pressure broadening of the oxygen line from foreign gas molecules in the sample mixture.
Cross sensitivity is caused by the pressure or Lorentz broadening of the oxygen absorption line due to their collision with other gas molecules. These collisions subtract or add a little energy to the natural or the Doppler-shifted energy of transition. The absorption amplitude is reduced at the center frequency and is increased at adjacent frequencies, broadening the spectral absorption line. This effect is increased as the collision frequency or pressure is increased. The peak absorption amplitude is also affected by the type of molecule colliding with the oxygen molecule absorbing the light. Large CO2 or N2O molecules interact more strongly with oxygen than argon atoms, for example, and couple translational energy to the quantum state of oxygen more efficiently. For example, the oxygen absorption line is about 3% wider in air, due to pressure broadening with nitrogen, than in pure oxygen. Without correction for cross-sensitivity, certified mixtures of 50%O2 / 50%CO2 or 50%O2 / 50%N2O read oxygen as being only 45%. Argon/oxygen mixtures without correction would read higher oxygen concentration.
Cross sensitivity customarily is corrected by measuring the foreign gas concentrations independently and computing a correction factor. By measuring the pressure and the line-broadening independently Oxigraf is able to correct to within 0.2% for cross-sensitivity without reference to foreign gas types or concentrations.
|
-
|